Agenda
Executive Summary
Problem Statement
Value Proposition
Product: Enzyme Replacement Therapy
Why Now: Market Opportunity
Business Model
Competitive Landscape
Go-To-Market Strategy
Team Overview
Traction & Milestones
Fundraising Goals
Overview
Executive Summary
Transfection Success
- We have delivered into/transfected human brain neuronal cells with a surrogate for GCase, using GFP. This marks a significant milestone in demonstrating the feasibility of our approach.
- Initial tests indicate that our transfection method maintains cell viability while effectively delivering the enzyme replacement therapy’s genetic material.
- This successful transfection is a crucial step towards proving the mechanism of action for GCase therapy in human neuronal cells.
Regulatory Pathway
- The FDA expects both successful delivery/transfections into human cells and delivery into a mouse model to proceed towards submission for approval.
- Our study design is structured to meet regulatory requirements, ensuring a smoother pathway to clinical trials.
- By securing funding for these studies, we can expedite our timeline and build credibility of our therapy in the eyes of potential investors and regulators.

Market Potential
- Parkinson’s disease affects millions globally, with limited effective treatments currently available, creating a significant market opportunity for our therapy.
- Our approach targets the root cause of the disease, positioning us favorably within the competitive landscape of neurological treatments.
- With initial funding, we can position ourselves as players in this space, opening doors for larger investments and partnerships.
Our innovative approach to treating Parkinson’s disease through enzyme replacement therapy shows promise and requires initial funding for mouse model studies. The FDA expects successful transfections in human cells and delivery into a mouse model as prerequisites for advancing towards regulatory approval.
Problem Statement
Parkinson’s disease is a progressive neurodegenerative disorder that currently has no cure. There is an urgent need for effective therapies to address the debilitating symptoms and underlying causes of the disease.
Understanding the Challenge of Parkinson’s Disease
• Parkinson’s disease affects approximately 10 million people worldwide, leading to significant disability and reduced quality of life.
• Current treatments only address symptoms, leaving a gap in curative options for patients.
• The role of glucocerebrosidase (GCase) enzyme deficiency in Parkinson’s pathology highlights a potential therapeutic target.
• FDA expects the demonstration of successful transfection in human brain neuronal cells and subsequent delivery in animal models for approval.
• Our initial studies utilizing a GFP surrogate for GCase will validate our approach before moving to clinical applications.
Total Economic Burden of Parkinson’s Disease (PD) Exceeds $50 Billion in the US Annually
• Opportunities exist to reduce this growing PD burden with pharmaceutical therapies
• Prescription medicines contribute only 16% ($4B) to the Direct Medical cost
• Current medicines treat symptoms but are not disease modifying
• Disease modifying therapies can reduce both medical and other indirect costs
A New Generation of Disease Modifying Medicines
Deliver Gene Therapies to Human Brain with AAV
Improving Delivery of Genetic Medicines to Human Brain is Possible—AAV technology
• Demonstration shown is with modified AAV delivery systems
• These viral systems have a host of drawbacks•
Demonstration shown is with modified AAV delivery systems
Compared to Other Cells, Neurons are Difficult to Treat
GoldenNeuro is Validating Materials in Neuronal Cells Lines
Prior to Testing in Mice
Gene Delivery to a Neuronal-Cell Line is Low
Compared to Control an Non-Differentiated Cells
- Gene delivery tested by using Organic Complex delivery of GFP plasmid DNA
- •Retanoic acid induces neuroblastoma cells into dopaminergic-neuron like cells
- •Parkinson’s disease affects dopaminergic-neuron function
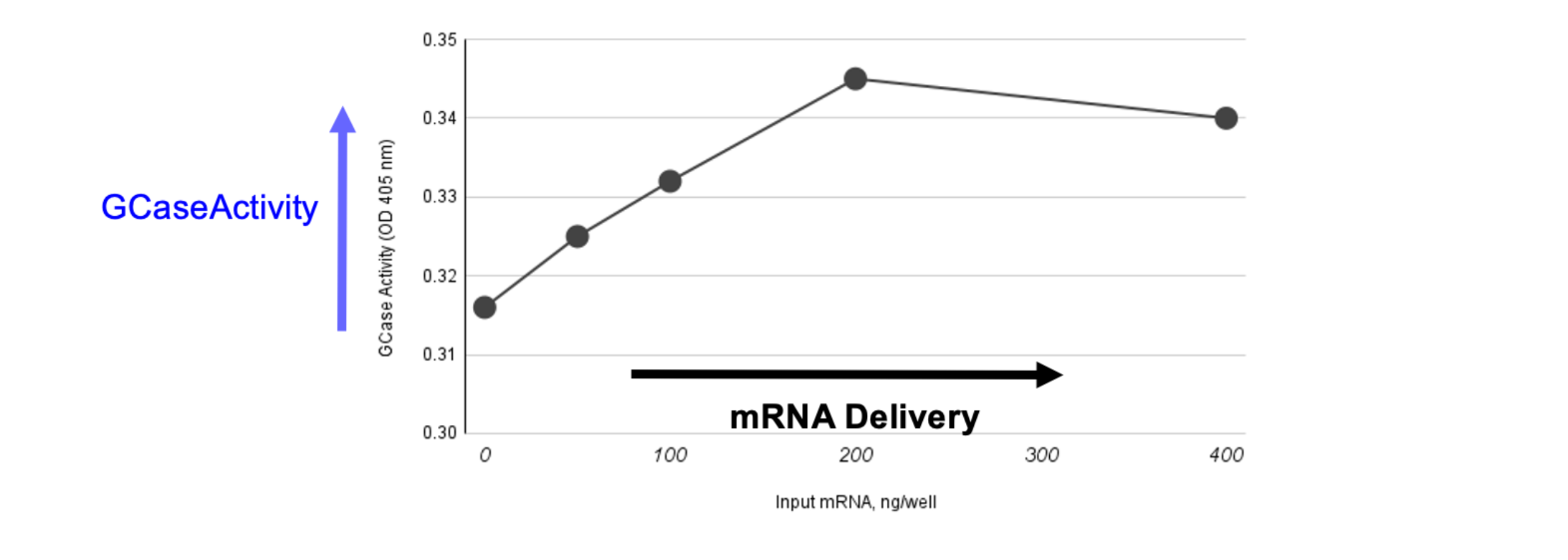
GoldenNuero has Increased Theraputic Protein Levels in Cells
using Non-viral Delivery of GCase mRNA
- Elevating Gcase activity in neurons slows Parkinsons Disease
- Neurons are difficult to deliver genetic medicines into (this example utilized surrogate ovarin cells- an easy to transfec host)
- lTo realize the disease modifying benefits of these medicines imrovements to neuron delivery are very desirerable
Value Proposition
Value Proposition
01
Innovative Therapy Approach
We are pioneering enzyme replacement therapy to target the root cause of Parkinson’s disease, potentially reversing neurodegeneration.
02
Transfection Success
Our successful transfection of human brain neuronal cells demonstrates efficacy, which is crucial for advancing to mouse model studies.
03
Pathway to FDA Approval
By establishing safety and efficacy through rigorous testing, we aim to secure FDA approval and bring our therapy to market.
Product Overview
Product: Enzyme Replacement Therapy
01
Mechanism of Action
Our therapy works by delivering functional GCase to neurons, addressing the enzyme deficiency that leads to neurodegeneration in Parkinson’s disease.
02
Surrogate Use
Initial studies will employ GFP as a surrogate for GCase to demonstrate the efficacy of our delivery system and transfection success.
03
Transfection Capability
We have successfully transfected human brain neuronal cells, a critical step required for FDA submission and validation of our approach.
04
Mouse Model Integration
Following successful transfection, we will transition to mouse models to further validate our therapy’s effectiveness and safety, aligning with FDA requirements.
05
Regulatory Pathway
Our product is designed to meet FDA criteria for enzyme replacement therapies, setting a clear path for clinical trials and eventual approval.
Business
Business Model
Funding Sources
- Strategic partnerships with biotech companies for shared development costs.
- Pursuing government and private grants aimed at rare disease research.
- Milestone-based investments from venture capitalists contingent on achieving specific research goals.
- Crowdfunding initiatives to engage the community and raise awareness.
- Collaborations with academic institutions for shared resources and expertise.
Revenue Streams
- Projected revenue from licensing agreements once FDA approval is obtained.
- Potential for partnerships with pharmaceutical companies for distribution and marketing.
- Long-term revenue from sales of GCase treatment once on the market.
- Subscription models for ongoing patient support and monitoring services.
- Potential expansion into related therapies for neurodegenerative diseases.
Our business model focuses on strategic partnerships, grant funding, and milestone-based investments to finance the development of our enzyme replacement therapy. This approach minimizes risk and maximizes the potential for successful commercialization and FDA approval.
Market Analysis
Competitive Landscape
Pros
- Our therapy offers a novel approach that targets the underlying cause of Parkinson’s disease.
- Significant unmet need exists in the market, with few effective treatments currently available.
- Potential to establish strong partnerships with key stakeholders in the biotech sector.
Cons
- Competition from established players with robust pipelines and funding.
- Regulatory hurdles may delay our entry into the market.
- Market acceptance of new therapies can be unpredictable and influenced by physician and patient preferences.
Strategy
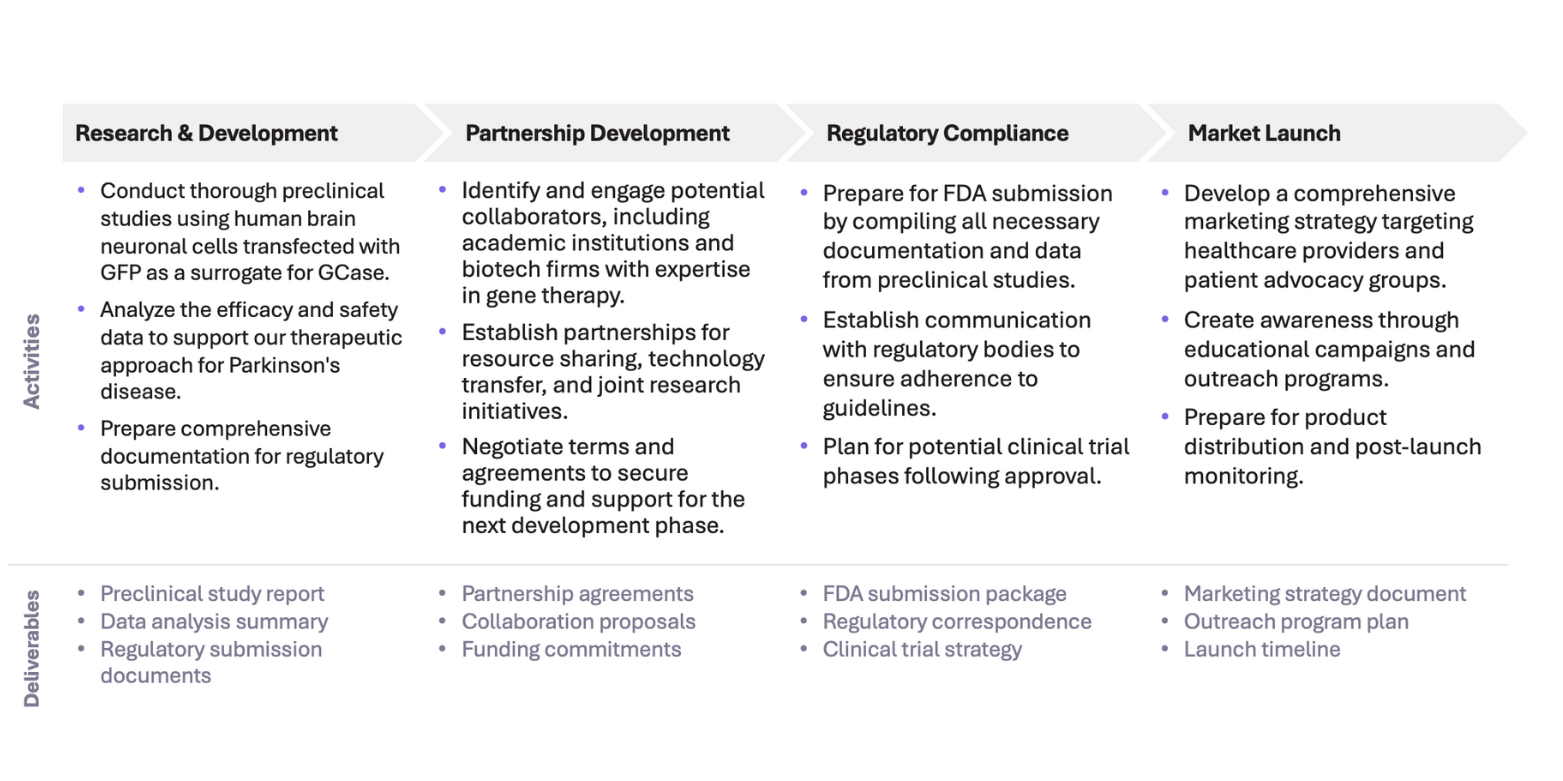
Go-To-Market Strategy
Our Go-To-Market Strategy outlines a clear path to launch our enzyme replacement therapy for Parkinson’s disease. By leveraging strategic partnerships, targeted marketing, and regulatory compliance, we aim to ensure a successful entry into the market and achieve FDA approval efficiently.
Team Overview
Our team consists of experts in neuroscience, gene therapy, and regulatory affairs. With diverse backgrounds, we are well-equipped to advance our program toward FDA approval.

Dr. Alice Thompson
CEO

Dr. Mark Johnson
CTO

Sarah Lee
Head of Research

James Brown
Lead Scientist

Emily White
Clinical Affairs Director

David Green
Regulatory Affairs Specialist

Laura Black
Project Manager

Tom Harris
Business Development Manager
Mouse model—ICV Injection
Kai Zhou, Jinming Han, Yafeng Wang, Yaodong Zhang, Changlian Zhu. Front. Mol. Neurosci., 25 October 2022
Sec. Methods and Model Organisms
Volume 15 – 2022 | https://doi.org/10.3389/fnmol.2022.988914

Preclinical
Expected Progression Path of ICV Injection Animal Models
Progress
Traction & Milestones
Human Neuronal Cell Transfection
95%
GFP Surrogate Successfully Delivered
100
Preclinical Mouse Model Study Initiation
Q2 2024
FDA Submission Target Date
Q4 2025
Funding
Fundraising Goals
Initial Funding Requirement
- We are seeking $40,000 in initial funding to support our critical preclinical studies.
- These funds will be allocated primarily for transfection experiments in human neuronal cells and mouse model studies.
- The funding will also cover necessary operational costs, including lab materials and personnel.
Use of Funds
- 70% of the funds will be dedicated to laboratory experiments and mouse studies.
- 20% will be allocated for personnel costs, including hiring experienced researchers.
- 10% will be reserved for administrative and overhead expenses to ensure efficient project management.
Projected Milestones
- Completion of transfection studies in human neuronal cells within 6 months of funding.
- Initiation of mouse model studies within 12 months, followed by analysis of results.
- Aim to submit our findings to the FDA for review within 18 months, paving the way for future investments.
Long-Term Vision
- Successful completion of these studies will attract larger investments needed for clinical trials.
- Our ultimate goal is to bring a transformative therapy for Parkinson’s disease to market, benefiting patients globally.
- Establishing a robust pipeline will position us strongly within the biotech industry for future projects.
Investor Benefits
- Investors will play a critical role in advancing a promising therapeutic approach for Parkinson’s disease.
- Potential for significant financial returns as we progress toward FDA approval and commercialization.
- Opportunity to be part of an innovative solution addressing a major unmet medical need.
To successfully advance our program toward FDA approval for the treatment of Parkinson’s disease through enzyme replacement therapy, we are seeking initial funds to support critical studies. These funds will enable us to conduct essential mouse model studies and transfect human neuronal cells, which are crucial for our FDA submission process.
Contact us
Lorem ipsum dolor sit amet, at mei dolore tritani in his nemore temporibus consequuntur, vim ad prima vivendum consetetur. Viderer feugiat at pro, mea.

